Research I 11 June 2023
Phase 2 kick off
Phase 1 completing and now moving to Phase 2 – analysis
nd of phase 1 resulted in the collection of all interventional studies (phase 2,3 or 4) in each disease area. Obtaining the data from either the clinical trial registry, ClinialTrials.gov or EU Clinical Trials Register. Additional from the clinical trial data, the publications from the studies were also gathered.
In total collecting over 39394 studies from ClinicalTrials.gov and 69171 studies from EU Clinical Trial register.
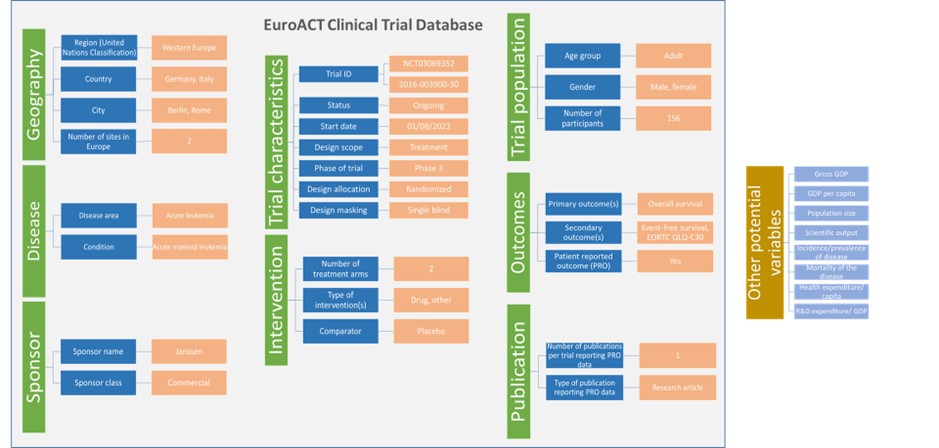
The following parameters have built up the EuroACT Clinical Trial Database:
Next steps
Research question who will shape the data analysis plan need to be drafted and agreed within the EuroACT patient community. The project will run a workshop with the community to nail down those questions and reach a consensus on what matters the community wants to get answers on already considering the future phases such as phase 3, generating the evidence.
Reach out if you have any questions on the project!
ABOUT THE PROJECT
EuroACT is a research project initiated by WECAN and the European Hematology Community. The project aims to understand the clinical trial landscape in the European region, based on data extracted from all relevant European clinical trial registers. Data from the past five years will unveil differences where clinical trials have been run in European countries and will describe how and which patient-reported outcomes (PROs) and quality-of-life (QoL) instruments have been used in clinical trials.


