Research I 7 March 2025
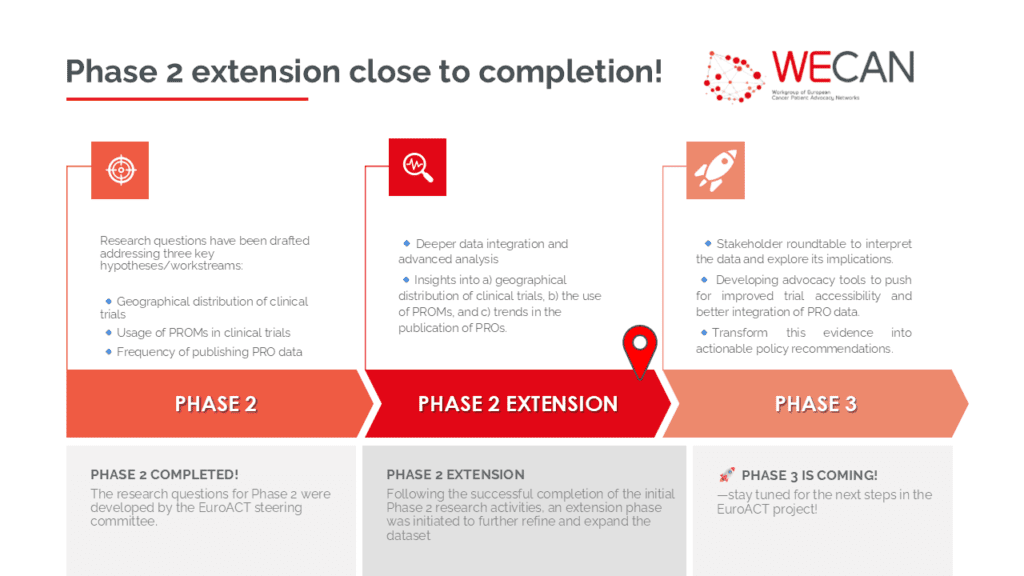
Phase 2 extension close to completion!
- In this phase, we updated details on the geographical distribution of clinical trials, the use of Patient-Reported Outcome Measures (PROMs), and trends in publishing Patient-Reported Outcomes (PROs).
- These findings enhance our understanding of clinical trial accessibility and highlight the importance of patient-reported data in research.
ABOUT THE PROJECT
EuroACT is a research project initiated by WECAN and the European Hematology Community. The project aims to understand the clinical trial landscape in the European region, based on data extracted from all relevant European clinical trial registers. Data from the past five years will unveil differences where clinical trials have been run in European countries and will describe how and which patient-reported outcomes (PROs) and quality-of-life (QoL) instruments have been used in clinical trials.


