Research I 11 June 2023
Research questions of phase 2 established
Where are we at?
Research questions have been drafted by the steering committee and have been reviewed and entire oncology and hematological community ensuring that the data outcomes are suited to their needs and answer questions they have around the three main hypothesis/workstreams:
EuroACT Phase 1 Overview
The research questions are established as follows:
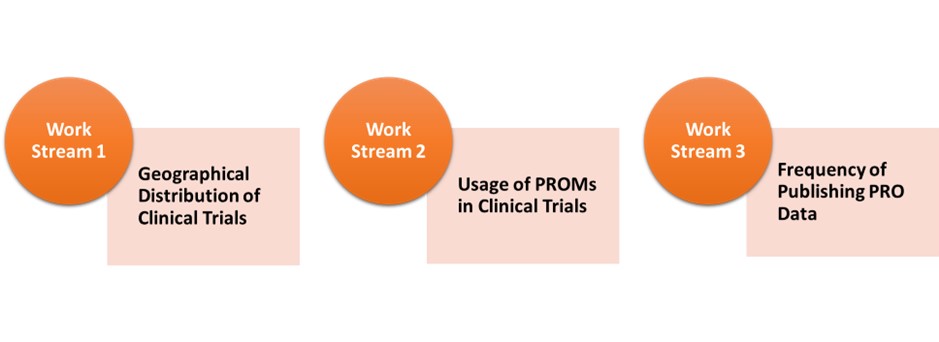
Geographical Distribution of Clinical Trials
- Q#1: Investigating regional and country differences in clinical trial activities across disease areas
- Objective:
- To analyse and compare the number of clinical trials and clinical trial sites across different regionsm of Europe and within countries of those regions, covering all disease areas collectively and each individual disease area under investigation.
- Objective:
- Q#2: Exploring the influence of socioeconomic and scientific factors on the country differences in clinical trial activities across disease areas
- Objective:
- To examine the impact of selected socioeconomic and scientific factors on the variation in the number of clinical trials and clinical trial sites across countries, considering all disease areas collectively.
- Objective:
- Q#3: Assessing the impact of study population age group on the country differences in clinical trial activities across disease areas and conditions
- Objective:
- To explore how the age group of the study population (adults vs. children/young adult) influences the number of clinical trials and clinical trial sites across countries, for all disease areas and specifically for 2-3 pediatric conditions identified by the EuroACT community.
- Objective:
- Q#4: Analysing the influence of study sponsor type on the regional and country differences in clinical trial activities across disease areas
- Objective:
- To explore how the study sponsor type (commercial vs. non-commercial) influences the number of clinical trials and clinical trial sites across different regions of Europe and within countries of those regions, covering all disease areas collectively.
- Objective:
- Q#5: Assessing the influence of disease rarity on the regional and country differences in clinical trial activities across selected rare diseases
- Objective:
- To assess how the rarity of a disease affects the distribution of clinical trials and clinical trial sites across different regions of Europe and within countries, for 2-3 rare diseases identified by the EuroACT community.
- Objective:
Usage of PROMs in Clinical Trials
- Q#1: Geographic variability in PROMs utilization in clinical trials across disease areas
- Objective:
- To assess and compare the utilization of PROMs in clinical trials across various European regions and within countries of those regions, covering all disease areas collectively and each individual disease area under investigation.
- Objective:
- Q#2: Investigating the influencing factors of PROMS usage in clinical trials across disease areas
- Objective:
- To examine the impact of selected factors on PROMs usage in clinical trials, considering all disease areas collectively.
- Objective:
- Q#3: Evaluating the nature of outcomes assessed by PROMs in clinical trials across disease areas
- Objective:
- To evaluate the characteristics of PROMs used in clinical trials, covering all disease areas collectively and each individual disease area under investigation.
- Objective:
- Q#4: Characterizing PROMs utilized in clinical trials across disease areas
- Objective:
- To evaluate the types of outcomes PROMs are used to measure in clinical trials, covering all disease areas collectively and each individual disease area under investigation.
- Objective:
Frequency of Publishing PRO Data
- Q#1: Investigating the publication rate of PROs in clinical trials across disease areas
- Objective:
- To evaluate the rate at which PRO results from clinical trials are published, covering both a collective analysis across all disease areas and detailed analyses for each individual disease area. This analysis also includes comparing the timing of publication for the first manuscript and the first published PRO data in completed trials within each disease area.
- Objective:
- Q#2: Exploring the influencing effect of clinical trial characteristics on the publication rate of patient reported outcomes (PROs) in clinical trials across all disease areas
- Objective:
- To assess the relationship between selected clinical trial characteristics and the publication rate of PRO results, considering all disease areas collectively.
- Objective:
- Q#3: Characterizing publications reporting PRO data from clinical trials across disease areas
- Objective:
- To assess the publication types, access levels, and scientific impact of articles reporting PRO data from clinical trials, both collectively across all disease areas and individually within specific disease areas under investigation.
- Objective:
Next steps
Based on the research question established the data analysis is currently being run and expected to be completed by end of July. The following steps is the preparation and laying the tools to drive the group discussions in roundtables and workshop with different stakeholders to address the data outcomes and generate the evidence on this understanding what the data means and what it can be used for. Phase 3 is coming!
Reach out if you have any questions on the project!
ABOUT THE PROJECT
EuroACT is a research project initiated by WECAN and the European Hematology Community. The project aims to understand the clinical trial landscape in the European region, based on data extracted from all relevant European clinical trial registers. Data from the past five years will unveil differences where clinical trials have been run in European countries and will describe how and which patient-reported outcomes (PROs) and quality-of-life (QoL) instruments have been used in clinical trials.


